Joint Research Team of KORDI and SNU Sheds New Light on Function of Zur, a Protein that Regulates Heavy Metal Concentrations in Cells
- HITS : 7439
- Date : 2011-03-09
A joint research team including Dr. CHA Sun-shin of the Republic of Korea Ocean Research and Development Institute (KORDI; President Kang Jung-keuk) and Professor ROE Jung-hye of Seoul National University (SNU) shed light on a previously unknown functional principle of Zur proteins, which regulate the concentration of zinc in bacteria.[O1]
Zur, or zinc uptake regulator, is a zinc-responsive tranxss-scription factor and refers to proteins that detect and regulate zinc concentrations in cells. Tranxss-scription factors are protein complexes that regulate the expression of genes.
Metals such as iron, zinc, copper, and nickel are essential to the survival of bacteria, but an excess of these metals in bacteria may be deadly to them. This is why bacteria have a system to maintain the concentrations of metals and sense change in them.
Zur proteins also detect change in the concentration of zinc in bacteria and thereby keep zinc homeostasis within an extremely narrow range to help the bacteria survive.
The joint research team confirmed through a high-definition three dimensional structure of Zur that Zur has three zinc-binding sites?named C-site, M-site, and D-site. The M-site’s ability to regulate tranxss-scription activation had been known through previous research, but it was this joint research that shed light on the D-site’s regulation ability.
The research team also proved that Zur consecutively reacts to changes in the zinc concentration by using two zinc-binding sites. It had been known that consecutive regulation of gene expression in cells depending on the degree of stresses are generally regulated by multiple tranxss-scription factors, but this research found that that may happen through a single tranxss-scription factor having two stress sensors with different sensitivity.
The findings of the research are expected to further the development of antibiotics that battle pathogenic bacteria, which infect the human body and cause disease, by limiting their use of metal and thereby killing them.
“If comprehensive study is successfully carried out about a range of heavy metal-responsive tranxss-scription factors that have similar three-dimensional structures to that of Zur, it will be possible to develop new antibiotics that limit use of metal by pathogenic bacteria,” says Dr. CHA Sun-shin of KORDI.
The research findings were published through the online edition of the Proceedings of the National Academy of Sciences (PNAS Online), a world-renowned comprehensive journal of sciences, as of March 7.
The joint team of Dr. CHA and Prof. ROE also conducted research on heavy metal sensing and DNA recognition functions of Nur, or nickel uptake regulator, which is a tranxss-scription factor that regulates the nickel concentrations in cells, and they published the findings in 2009 through Nucleic Acids Research, a prominent international journal in biological science.
The Proceedings of the National Academy of Sciences is published by the National Academies of the US and enjoys a global reputation in biology, physics, and social sciences, among other fields. Its first issue was published out in 1914.
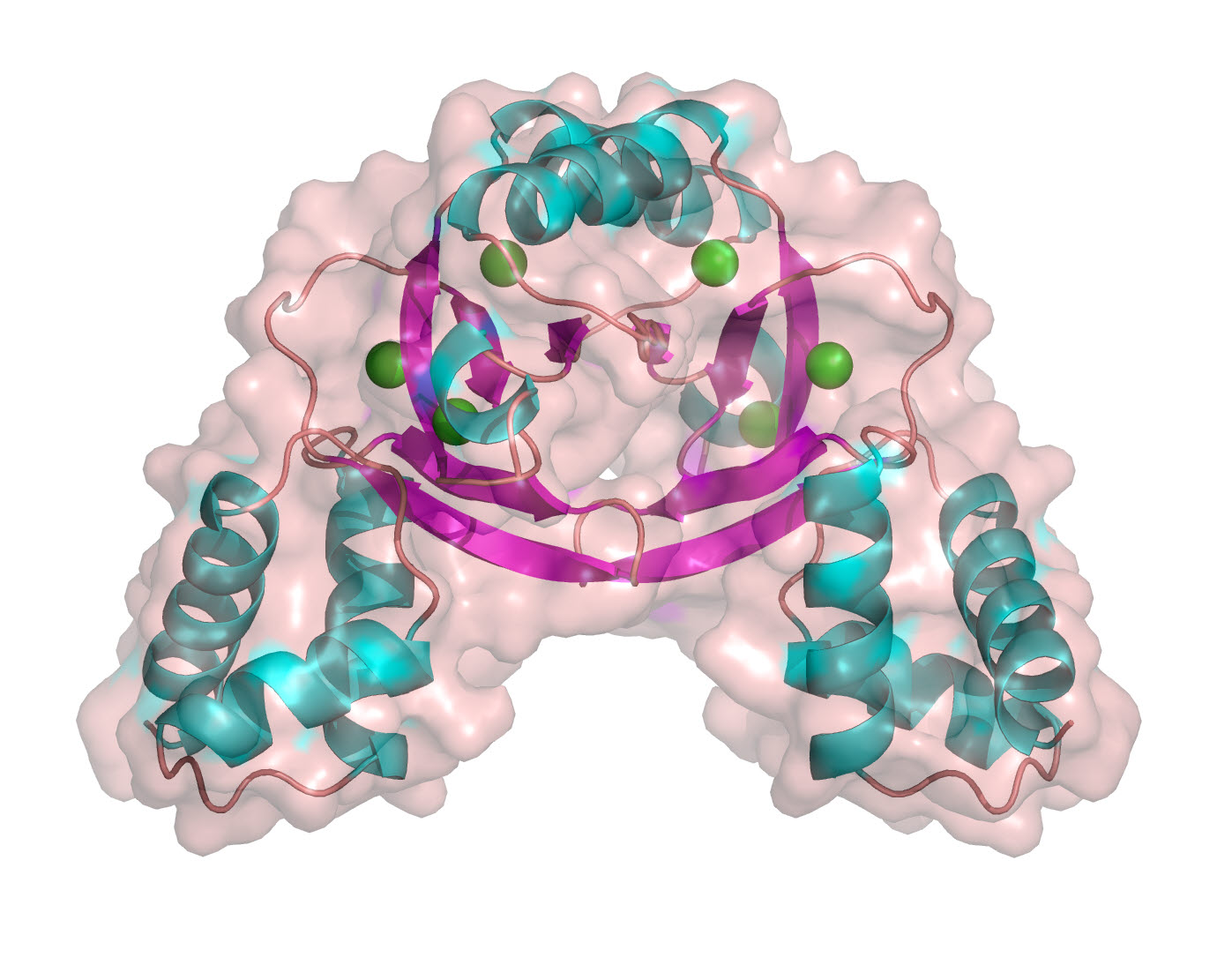
▲ Three dimensional structure of a Zur protein
- Content Manager :
- Last Update : 2024-01-31